 Hsueh-Chia Chang, back to work on research adapted to improve virus detection. Photo by Matt Cashore ’94
Hsueh-Chia Chang, back to work on research adapted to improve virus detection. Photo by Matt Cashore ’94
While campus was shut down during the second half of the 2020 spring semester, members of Hsueh-Chia Chang’s chemical and biomolecular engineering lab were hard at work on a specialized COVID-19 test. As the pandemic worsened throughout that spring and into the summer, graduate students Liao Chen and Chenguang Zhang, along with technician Ceming Wang, adapted the Chang lab’s existing work to meet the testing needs of the worldwide viral outbreak.
The sense of urgency became personal for Chang when he contracted the virus in November, after most students and faculty had returned to campus. He spent 11 days in the hospital, five in critical condition. While he eventually recovered, his experience strengthened his resolve to develop a rapid alternative to current methods of virus screening.
“After I got out of the hospital, I decided that that’s exactly what I’m going to do,” Chang said. “I thought, forget about writing papers, and fortunately I have a good group that shares my passion.”
Earlier in the year, his students set out to improve the speed and accuracy of virus testing, designing a process that would eliminate the need for outsourcing to a lab. They also hope to do more with less; that is, detect low levels of virus to catch new cases before people reach the height of infectiousness. Chang, the principal investigator, hopes this new way of testing for novel viruses in their early stages of spreading will help mitigate the next pandemic.
Chang’s research group typically focuses on detecting cancer biomarkers. Many of these biomarkers are carried in tiny, bubble-like vessels called exosomes, which are excreted from cells in a process called cell signaling. Exosomes contain bits of DNA, RNA or protein that can be used to identify the kind of cell they came from.
Luckily, the structure and size of exosomes are not too different from the key player of the COVID-19 pandemic: the viral pocket of SARS-CoV-2. Recognizing the similarities between the two kinds of nanoparticles, Chang steered his lab to adapt existing research to focus on virus detection.

Most COVID-19 tests are based on a process called PCR, known in chemistry and biology labs as polymerase chain reaction. During PCR, specialized proteins latch on to fragments of DNA or RNA and imitate cells’ natural process of genetic replication. Once enough of the genetic material has been produced, the identity of the fragments can be determined.
For coronavirus testing, fragments of RNA are isolated from mucus or saliva samples. Special PCR ingredients latch on to pieces of RNA that code for the coronavirus spike protein, revealing the presence of the virus if it is there. It’s as if the PCR proteins are digging out a buried treasure and yelling, “Hey! It’s over here!” and providing a magnifying glass to better see the RNA. In this case, they’re looking for indicators that coronavirus particles are present.
PCR ingredients can be substituted or supplemented to single out a virus other than COVID-19 or multiple viruses at once. Going after multiple viruses is called multiplex screening.
The issue with most PCR testing, however, is that it must be outsourced to a lab with equipment powerful enough to replicate a lot of RNA from a tiny human sample. Chang’s lab set out to develop a PCR testing process that could be done quickly and on site, at a pop-up testing clinic, for example.
The researchers took a different approach to PCR amplification than what’s widely used. Chen and Chang optimized a method of “digital droplet” PCR (ddPCR).
Here’s how it works: A “master mix” of PCR components is prepared, and the collected COVID-19 testing sample is added to this mix. This combined solution is transferred into a mixture of oil and other chemicals, which yields several hundred stabilized droplets that each contain a small amount of the PCR mixture. This droplet panel then goes into a machine that activates the process of replication.
As the RNA fragments multiply, they fill the oil droplet which houses them, eventually ballooning the droplets to a size large enough to be seen without a microscope. During the replication period, which takes only two hours, a glowing probe is added to the new strands, making the target RNA glow blue under a blue-light filter, visible to the naked eye.
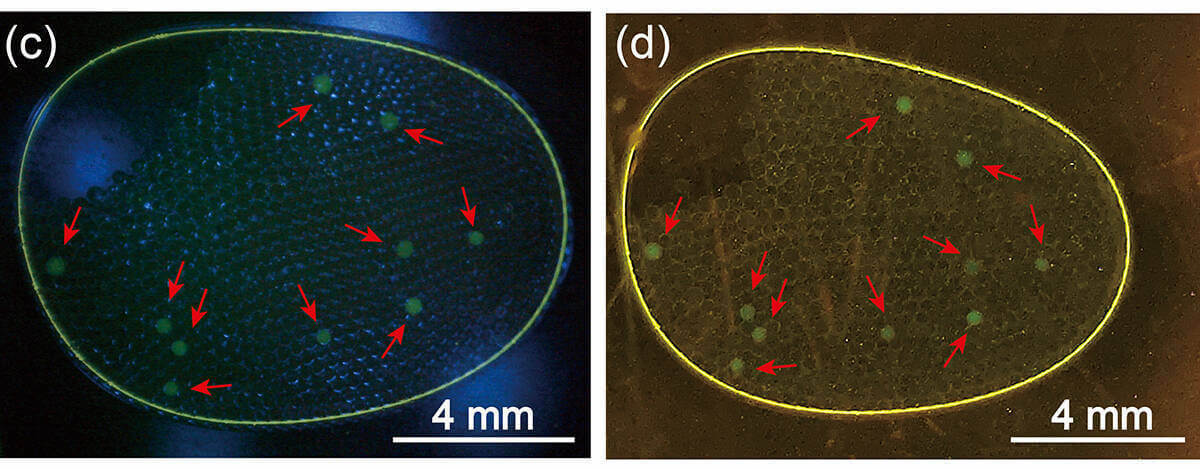
This streamlined process can be completed using materials and procedures most biology and chemistry labs already have on hand, said Chen, the lead researcher. That makes it a viable and inexpensive option for research universities. “Relative to other methods out there, it’s so easy.”
Because RNA can be directly amplified from the original biological sample, the Chang ddPCR is also ideal for detecting a very small amount of virus in pooled samples, like municipal sewage, to track community spread. As a result, it is better suited for detecting infections in the early stages of a pandemic. Chang is optimistic about its future potential.
Chang’s lab has set out to refine their testing method and commercialize it, creating a prototype that can combine testing samples, form droplets, run the PCR procedure and examine the post-amplification droplets under a blue-light filter, all in one compact machine. The group also hopes to develop a multifaceted viral panel to screen for many viruses, and variants within those viruses, all at once.
Successful development of this technology would provide tools to rapidly map and isolate new viral cases within hours at the start of a pandemic. More people would have access to rapid tests without needing to go a lab or hospital with fancy equipment.
Fast, accessible testing will be important to fighting the next pandemic, Chang told Notre Dame News. He hopes the effects of the COVID-19 pandemic will lead to policy changes that invest more in preventative care, including developing tests before there is an immediate need.
“Screening tests are fine, screening tests are important too,” he said. “But you have to prevent the next outbreak. It’s going to happen. And I can tell from personal experience, it is not something you want to go through.”
Erin Fennessy, a senior biochemistry and French major with a minor in journalism, ethics and democracy, was this magazine’s summer 2021 intern.